What is a Clinical Trial?
Clinical trials, also called clinical studies or clinical research, advance medical knowledge in which one or more human subjects are prospectively assigned to one or more interventions to evaluate the effects of the interventions on biomedical or behavioral health-related outcomes and procedures using a rigorous scientific method.
Clinical trials move science forward as the safest and most effective way to discover and test new devices, treatments and ways to perform medical procedures and interventions. Subjects in clinical studies are mostly patient volunteers that take the treatment and provide their outcomes for evaluation, under a strict, ethically cleared, protocol.
Phases
Clinical trials are conducted in phases to secure and make a more efficient procedure:
- In Phase I clinical trial, drugs or devices are evaluated within a small group to identify possible side effects and determine dosage.
- A Phase II clinical trial is designed to measure whether the treatment is effective while continuing to evaluate safety. Some patients may receive differing treatment protocols.
- A Phase III clinical trial evaluates and compares the intervention against the current standard of care.
- A Phase IV clinical trial comes if Phase III proves successful and a new treatment is approved for the general public, researchers will continue to collect data from clinical trial participants.
Today, there is a lot of discussion about clinical trials, due to the Covid-19 vaccines, therefore, we selected a recently published scientific work from The Lancet that presents ongoing and future COVID-19 vaccine clinical trials: “Ongoing and future COVID-19 vaccine clinical trials: challenges and opportunities“.
Healthentia in Clinical Trials
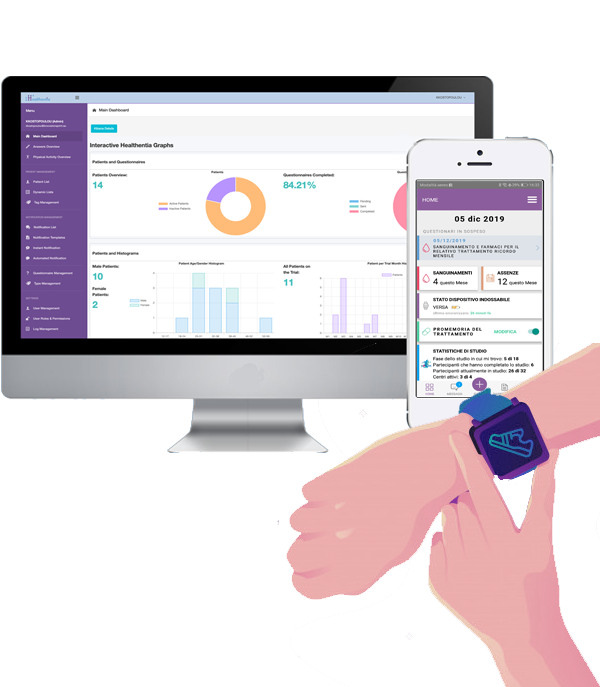
Healthentia is an eClinical platform that captures Real World Data for decentralized Virtual Clinical Trials while utilizing digital composite biomarkers to offer smart services under the context of eHealth and DTx.
At Innovation Sprint we support the digital transformation of the Lifesciences and Healthcare industries, which we have seen in the last year a rapid shift to this technology path. Our scope is to improve clinical and healthcare processes and to develop personalized healthcare solutions.
For more information and DEMO presentation of Healthentia solution, you can contact us at info@innovationsprint.eu.