JOIN OUR VISION
Boosting digital transformation in Clinical practice & Life Sciences with MedTech

Healthentia is a Medical Device Class Ila

EUDAMED registration: Healthentia v4
Read more about medical modules and device functionalities that are covered under the CE mark.
Software as a Medical Device (SaMD)
HEALTHENTIA is a medical decision support software developed by INNOVATION SPRINT and intended to monitor, offer virtual coaching services and generate automatic alerts regarding events, based on Real World Data.
Ιntended Use: Healthentia App is part of Healthentia solution, a software intended for: a) the collection and transmission of physiological data including heart rate, blood pressure, oxygen saturation, and weight directly to care providers via automated electronic means in combination with validated IoT devices; b) the visualization (subjects-based dashboards) and the mathematical treatment of data (trends analysis, alerts) related to the monitored chronic disease subject’s physiological parameters; c) the transmission of patient’s outcomes and outcome scores related to patient’s health status, health-affecting factors, health-related quality of life, disease knowledge and adherence to treatment through validated questionnaires; d) the user (subject/patient) interaction with a conversational virtual coach for informative and motivational purposes, in order to support subject telemonitoring, decision making and virtual coaching.
Clinical benefits: The use of Healthentia provides objective inputs for healthcare professionals to support diagnosis, to highlight evolution of physiological parameters by trends analysis of the patient’s inputs, and to allow healthcare professionals to provide the same quality of care and safety as the standard of care.
Clinical Indications: Telemonitoring of chronic disease patients (such as heart failure, cancer, COPD, etc.)
Patient Target Groups: Chronic disease patients taking part of clinical investigation or a medical treatment.
Intended users: Telemonitored patients and their healthcare professionals.
Use environment and duration: Healthentia App can be used as Remote Patient Monitoring solution for patients that are released from hospital and have to follow a certain treatment. Duration depends on the study or intervention or patient's will.
For Instructions for use, IFUs (see from https://healthentia.com/saas-agreement/)
More information about the medical device is available here:
WHY HEALTHENTIA
An Integrated Solution
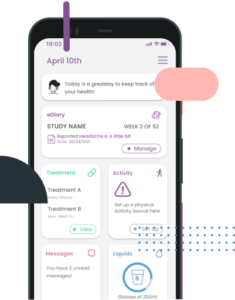
Healthentia App can be customized for different therapeutics and studies. It serves as a Companion app collecting data following a care pathway, supporting & coaching the patient.
A companion App
Equips patients to be agents of their own health outcomes and well-being
Clinical Dashboard
Enable hospitals to lower preventable readmissions and complications & improve quality of care and patient satisfaction
Experience & Expertise
Applications
Bringing value to Patients, Healthcare & Pharma
Digital Health
Remote Patient Monitoring
Disease Management
Digital Therapeutics (DTx)
Life Sciences
Decentralized Clinical Trials
Co-creation Patient Programs
