CATEGORY: Conference
SOURCE: 13th International Conference on Healthecare Informatics, July 22nd 2025, 10.1109/ICHI64645.2025.00109
Abstract:
I. INTRODUCTION
Respiratory diseases are considered as one of the major causes of global morbidity and mortality [1] and, as reported by the World Health Organization [2], Chronic Obstructive Pulmonary Disease (COPD) is the fourth leading cause of death worldwide. Due to symptoms such as chronic cough or breathing difficulty, this condition has a great impact on daily life and habits. Additionally, COPD is often accompanied by concurrent cardiac, psychological, or other comorbidities [3], which require specific and tailored care plans for patients, as well as frequent follow-up visits and care services. For these reasons, the health burden associated with this chronic condition is considered significant [4].
Patients with COPD may experience a worsening of symptoms, defined as an exacerbation [5], associated with acute systemic inflammation and clinical status changes [6]. Such events might be triggered by a specific event or other physiological causes [7], occur rapidly from hours to days [8] and often require medical consultations or even hospitalization with a consequent change in the regular treatment of the chronic condition [9]. In more serious cases, exacerbations can lead to death. For these reasons, a prompt identification of symptom worsening is of utmost importance in limiting the rise of potential moderate exacerbations while lowering the number of hospital admissions.
Since COPD is a chronic condition, patients usually adhere to a tailored therapy plan to be followed at home while they report their health status to doctors during follow-up visits. In this setting, timely identification of symptom worsening can only happen if patients are able to recognize any relevant change in their clinical condition and decide to call clinicians or seek additional hospital care. Self-management and remote monitoring and care might make the difference in the treatment of this pathology by having access to information such as the daily health status of a patient or specific symptoms related to COPD and concurrent comorbidities. Several studies have been published in the virtual coaching domain as well, with the aim to actively assist the patient in their disease self-management process [10][11].
The importance of early detection of COPD exacerbations is reported in other works, as in [10], [12] and [13]. Approaches that have been explored include self and remote monitoring, as well as symptom identification, with the use of daily questionnaires [14], or wearable devices [15], and the potential inclusion of prognostic models [16] in COPD patient care. The detection of exacerbations can rely on heterogeneous data, depending on a given modelling approach, and may include parameters such as Forced Vital Capacity (FVC) or Forced Expiratory Volume in 1 second (FEV1), as well as other clinical data, real-world data or self-reported symptoms. Digital tools and telemonitoring are presented in several works: in [17], the authors present a telemonitoring system along with models predicting COPD exacerbation, whereas in [18] daily remote monitoring of symptoms and k-means clustering is described. In [14], daily scores from questionnaires combined with spirometry, pulse oximeter and clinical data are used to identify the occurrence of a health-worsening event, whereas in [15] the authors describe an approach including the recorded heart rate. Emerging technologies, such as machine learning (ML) and artificial intelligence (AI) approaches for predicting COPD exacerbations are presented in different papers, e.g., the authors in [19] describe a decision tree forest classifier for the prediction of symptom worsening episodes, whereas a comparison of ML algorithms for acute exacerbation identification is presented in [20].
As described in [14], a challenge on the actual use of remote monitoring and digital tools is the large number of alerts, which may result in an unmanageable number of notifications and additional workload for healthcare professionals (HCPs). Moreover, low adherence, missing data and errors in data collection might affect the robustness and reliability of the output produced by the system.
To overcome these limitations, the REal-time data monitoring for Shared, Adaptive, Multi-domain and Personalised prediction and decision making for Long-term Pulmonary care Ecosystems (RE-SAMPLE) project (accessible at https://www.re-sample.eu/) has developed a digital framework to support both patients with COPD and Comorbid Chronic Conditions (CCCs) in self-management of their diseases and Healthcare Practitioners (HCPs) in providing personalised care through shared decision making. With the use of a mobile application, patients can be followed remotely by self-reporting any symptom changes and they can receive tailored suggestions to improve their lifestyle and enhance their disease awareness. Additionally, a clinical dashboard allows for patient monitoring by HCPs thanks to the direct access to information from several sources, including clinical records, answers to questionnaires, exacerbations and risk predictions.
The clinical protocol and the algorithm used in the RE-SAMPLE study to detect the start (referred to as onset) and end (referred to as offset) of a COPD exacerbation have been adapted from the COPE-III study in [21] and [22]. In these previous works, the authors aimed at introducing and evaluating an innovative treatment approach based on self-management and action plans, on the basis of self-reported data including the use of daily symptom diaries and the definition of a stable condition versus an exacerbation state. Additionally, other works implemented part of these clinical protocols into algorithms for digitized diaries within e-health solutions, showing high adherence rates and feasibility for use as part of COPD disease management [23][24]. However, further research is necessary for the full implementation, the validation and eventually the improvement of the COPE-III protocol, using richer data sources, and facilitating larger-scale studies.
Therefore, an algorithm has been implemented for the identification of exacerbations based on patient answers to questionnaires and daily interactions with the app. Preliminary results on the use of this algorithm in the RE-SAMPLE multicentric study are presented in this paper, along with a comparison with actual emergency accesses and hospitalizations from Electronic Health Records (EHR) as a primary validation scheme. Even if the model is designed to support CCCs, the analysis of algorithm performance for the concurrent presence of COPD and other pathologies will be described in another work. Details on the pilot sites infrastructure, as well as data collection processes, are beyond the scope of this paper and can be found in [25], [26] and [27].
In the following sections, a description of the algorithm used for the identification of exacerbation events is reported, along with the study protocol, data sources and the preliminary results obtained by its application in a real-world scenario. Conclusions and future directions are finally presented to outline the next steps in the validation of this approach.
II. METHODS
A. Data collection and management
In the RE-SAMPLE study, Healthentia SaMD (Software-as-Medical-Device) [28], is used to collect data from patients and enable their interaction with a virtual coach. Healthentia is a Class IIa medical device software intended for: a) the collection and transmission of physiological data including heart rate, blood pressure, oxygen saturation, and weight directly to care providers via automated electronic means in combination with validated IoT devices; b) the visualization (subjects-based dashboards) and the mathematical treatment of data (trends analysis, alerts) related to the monitored parameters; c) the collection of patient reported outcomes related to health-related quality of life, disease knowledge and adherence to treatment through validated questionnaires; d) the user (subject/patient) interaction with a conversational virtual coach for informative and motivational purposes, in order to support subject telemonitoring, decision making and virtual coaching. Healthentia consists of two parts: the smartphone-app for chronic disease patients and the clinical dashboard for HCPs [29].
In this study, HCPs monitor patients through the Healthentia clinical dashboard. It displays aggregated metrics including vital signs and activity levels and helps identifying trends. The system can automatically flag potential issues and calculate risk scores to help providers take timely action. It also includes tools for creating and sending custom health questionnaires to patients [30].
The Healthentia mobile app helps patients actively participate in their healthcare by collecting RWD. It connects with fitness devices – particularly Garmin trackers in the RE-SAMPLE study – as well as other health apps to gather information, while allowing patients to answer health questionnaires and track their treatment progress. A virtual coach provides personalized guidance and motivation based on the patient’s data [30].
The detection of exacerbations is based on the comparison between the symptoms reported by patients in What Are My Symptoms Questionnaire (USQ) during the enrollment visit and the ones self-reported on a daily basis via the multi-morbid Daily Symptoms Questionnaire (DSQ). This composite questionnaire consists of multiple sub-questionnaires, each addressing specific conditions such as COPD, Chronic Heart Failure (CHF), Anxiety, Depression and Ischemic Heart Disease (IHD) [22]. Patients complete the DSQ through the Healthentia mobile app. The DSQ is adaptive and can be dynamically adjusted based on patient responses and health status. Every morning patients receive one question about whether they have experienced any worsening of symptoms, the questionnaire is completed. If it is positive, they proceed to questions related to COPD symptoms, followed by assessments of anxiety and depression. The system then continues with condition-specific questions based on the patient’s reported comorbidities. If any worsening of symptoms is detected, patients are asked a final free-text question to reflect on potential contributing factors.
The daily score generated from the COPD symptoms questionnaire, part of the DSQ, is used by the algorithm to detect symptom fluctuations, forming the foundation for detecting COPD exacerbations. The scoring encompasses changes in major symptoms: breathlessness, sputum volume, sputum color, and minor symptoms: fever, cough and wheezing and is detailed in Table 1. Each day the algorithm checks the overall summed score; a moderate symptom increase is defined as a score of 10-110 and a significant symptom increase as a score of more than 110. This condition means that at least one of the major symptoms and one of the minor ones are reported as “Significantly more than usual”.
Table 1: COPD Symptoms questionnaire scoring used in exacerbation detection algorithm
| Options | Breathlessness | Sputum Volume | Sputum Color | Fever | Cough | Wheezing |
|---|---|---|---|---|---|---|
| Not more than usual | 0 | 0 | 0 | 0 (No) | 0 | 0 |
| Slightly more than usual | 1 | 1 | 1 | 10 (Yes) | 1 | 1 |
| Significantly more than usual | 100 | 100 | 100 | – | 10 | 10 |
| Overall score | Sum of all questions (0-330) |
B. Algorithm design
The algorithm design employs the COPE-III protocol [22] to systematically detect the onset and resolution of COPD exacerbations. An exacerbation is defined as a period of symptom deterioration, identified through at least two consecutive deviations for at least two COPD symptoms from baseline symptomatology. Criteria are also reported in [31] and [32].
At any given moment patients are classified into one of three mutually exclusive states: COPD Stable, COPD Exacerbation, and COPD Unstable. Transitions between states are dictated by DSQ responses and follow a structured multi-stage process, incorporating sub-state transitions necessary for automated system actions. These types of actions are sending app notifications (e.g., contacting their HCP for support) and/or additional questionnaires to patients, sending dashboard alerts to HCPs, and applying the tag of the current state to the patients.
The Stable state is the default state patients present upon enrollment in the RE-SAMPLE study. They receive the DSQ every morning, and their transition to another state depends on their COPD symptom scores. A score exceeding 110, which indicates the presence of a significant change of breathlessness, sputum volume change or sputum color and fever or cough and wheezing symptoms, triggers a transition to the Unstable state as defined in the COPE-III protocol. At this state if a patient does not answer DSQ for more than four consecutive days first a reminder is sent to the mobile app and finally a dashboard alert is sent to the HCP to contact the patient.
The Unstable state represents the first day of a significant COPD symptom deviation and is an intermediate state. The next day, if COPD questionnaire score is again higher than 110 the patient transitions to the “COPD Exacerbation Onset”-state. If the score is less than 110 the patient returns to the Stable state since 2 consecutive days of two significant symptom deviations are required to transition to Onset. Finally, if patients fail to complete the DSQ the following day, the system checks if 3 days have passed while in the Unstable state, and if this is true, patients return to the Stable state.
The Exacerbation state consists of the following sub-states: 1) the Onset state, that signals the first day of exacerbation, 2) the “Super”-states for ongoing monitoring of symptoms, and 3) the Offset state, that represents recovery and transition back to stability. These sub-states are only required for the systemic actions and the correct implementation of the algorithm. In the clinical dashboard patients in these states retain the status tag “COPD Exacerbation” as for HCPs the sub-states do not have any clinical value. The Onset state is bounded with some systemic actions: the patient receives a notification alert in the mobile app prompting them to perform a blood sample test and a dashboard alert is sent to the HCP via the Healthentia portal. Subsequent DSQ responses dictate transitions to Super state with no symptoms, Super state with moderate symptoms or Super state with significant symptoms. If the DSQ is not answered the following day, then significant symptoms deviation is assumed, and the Super state is assigned as such. If a patient remains in the Super state but stops answering for four consecutive days, a web alert is sent to the HCP.
The exacerbation ends when predefined conditions are met, transitioning the patient back to the Stable state. At this point, their status tag updates to Stable and they receive assessment questionnaires for further evaluation. The two conditions for the Stable transition are either 7 consecutive days with a score lower than 10 or 3 consecutive days with a score of 0 are observed which clinically represents the absence of symptoms or the presence of moderate changes in symptoms such as fever, cough or wheezing. These conditions are based on [22], [31] and [32]. If neither condition is met, the patient remains in exacerbation for a maximum of 30 days, assuming that no exacerbation can last longer. Following this approach, the minimum duration of exacerbation is 4 and maximum duration 30 days.
The described transitions and their conditions are visualized in the following figures:
- Fig. 1 demonstrates the conditions to transition from Stable state to Unstable
- Fig. 2 the conditions to transition from Onset to one of the Super sub-states
- Fig. 3 the conditions to transition from a Super state back to Stable Systemic actions can be either an app notification destined for the patient or a dashboard alert destined for the portal user.
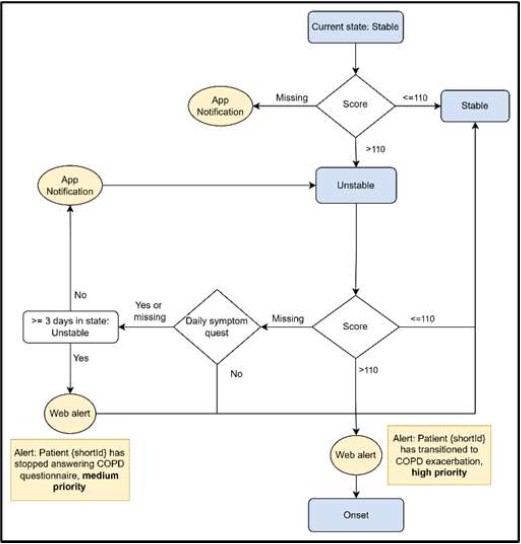
Fig. 1. Flow diagram showing conditions for transitioning from Stable state to Unstable state, actions related to each condition and specific score checking. Blue boxes represent states while yellow ones represent systemic actions related to a condition.
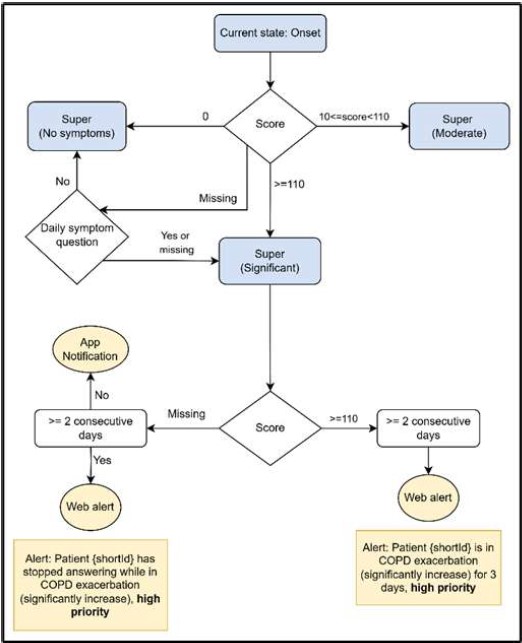
Fig. 2. Flow diagram showing conditions for transitioning from Onset to one of the Super sub-states: no symptoms, moderate or significant, systemic actions and specific score checking. Blue boxes represent states and the yellow ones represent systemic actions related to a condition.
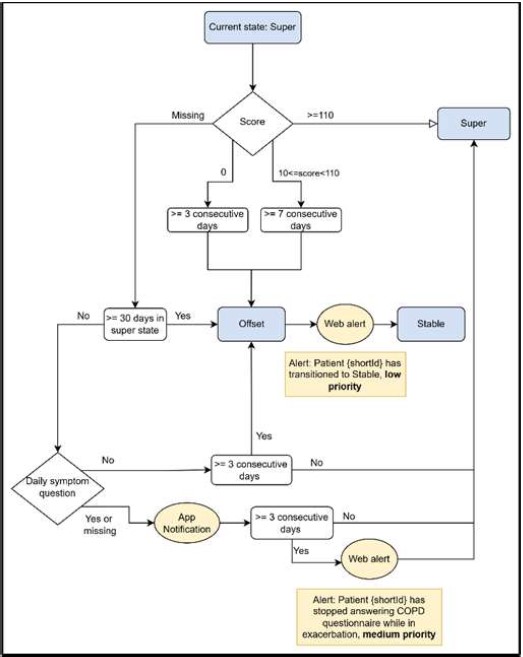
Fig. 3. Flow diagram showing conditions for transitioning from Super to Offset and finally back to Stable, systemic actions and specific score checking. Blue boxes represent states while yellow ones represent systemic actions related to a condition.
C. Study design
The RE-SAMPLE project includes both retrospective and prospective studies performed in three European pilot sites, the Fondazione Policlinico Agostino Gemelli IRCCS in Italy (GEM), the Stichting Medisch Spectrum Twente (MST) in the Netherlands and the Sihtasutus Tartu Ulikooli Kliinikum (TUK) in Estonia. RE-SAMPLE’s major objective is to increase the understanding of COPD and CCCs by creating a knowledge base of multimodal data from EHR, RWD collection, patient knowledge and guidelines. To reach this goal, the consortium designed and implemented a Virtual Companionship Program (VCP) for the patients and an Active Support Program (ASP) for HCPs using the Healthentia infrastructure and developing a dedicated dashboard and shared decision-making tools.
The retrospective studies have been focused on creating a COPD related knowledge base of patient phenotypes. The prospective studies instead included two sub-studies, an observational cohort study and an interventional study with the introduction of the VCP. The observational study has the objective of analyzing the predictors of exacerbations of COPD, as well as assessing technology adoption by patients and HCPs. Furthermore, a fine tuning of the various functionalities of the patient application, including e.g. the use of daily symptom diaries and answering questionnaires, has been included. The interventional study instead implements the VCP and the ASP programs and is still ongoing. The enrolled patients are diagnosed with COPD and concurrent CCCs such as CHF, IHD and diabetes, whereas patients with cognitive impairment or serious other diseases (including severe psychiatric illness or low survival rate) were not included in the studies to avoid confounding factors.
The implementation of the COPE-III protocol in a digital application has been integrated and tested since the beginning of the prospective studies while being adapted over time to account for low adherence rates and data availability. Additionally, the calculated exacerbation events are one of the main outcomes used as a disease deterioration indicator by the models trained in the federated RE-SAMPLE platform. Such models are trained on both hospital and self-reported data aiming to assess risk levels and provide relevant clinical explanations. Alerts and risk predictions related to exacerbation events feed the clinical dashboard that supports clinicians in monitoring the enrolled patients during the interventional study. Moreover, this approach fosters better self-management through the recognition of symptoms and actions suggested to patients. Therefore, evaluating the algorithm effectiveness in detecting COPD exacerbations through patient-reported data is crucial for several components of the project both technical and clinical.
D. Dataset and algorithm validation methodology
In total 232 patients fulfilled the inclusion criteria and participated in the RE-SAMPLE prospective study, with 55 patients dropping out. 30.6% of the entire cohort were female with a mean age of 71 ± 9.2 years. A more comprehensive overview of the cohort characteristics is reported in Table 2. Enrolled patient number differs slightly per pilot site, with GEM presenting 86 patients, TUK 75 and MST 71. Overall, enrolled patients demonstrated a mean actual usage of the tool up to 656 days, while patients at TUK appear to have been using the app for a longer time period. Data covering an approximately 3 years period from 2022-01-25 to 2025-02-14 has been extracted to analyse patient enrolment and compliance aspects, exacerbation number, frequency and duration overall and among pilot sites.
Table 2: General study characteristics across sites in Healthentia database. Categorical variables are reported as counts and percentages while numerical variables as mean values and standard deviations.
| Pilot Site | GEM | MST | TUK | Total |
|---|---|---|---|---|
| Enrolment status metrics | ||||
| Enrolled | 86 | 71 | 75 | 232 |
| Drop out | 5 (5.8%) | 31 (43.7%) | 19 (25.3%) | 55 (23.7%) |
| Actual usage period (days) | 648±204 | 642±267 | 679±369 | 656±284 |
| Demographics variables | ||||
| Sex | M: 53 (61.6%), F: 33 (38.4%), N/A: 0 (0.0%) | M: 35 (49.3%), F: 25 (35.2%), N/A: 11 (15.5%) | M: 62 (82.7%), F: 13 (17.3%), N/A: 0 (0.0%) | M: 150 (64.7%), F: 71 (30.6%), N/A: 11 (4.7%) |
| Age (years) | 74.5 ± 9.6 | 69.6 ± 12.9 | 69.2 ± 5.4 | 71.0 ± 9.2 |
The validation of the proposed algorithm outcomes follows an external validation scheme: firstly, we investigate the number of identified events, based on the self-reported symptoms, and secondly, we include EHR data on hospitalizations, emergency accesses and death events available in a dedicated COPD datamart. We report in detail the results on the GEM pilot site in the next section.
III. RESULTS
In this section we provide preliminary results on the dataset and the algorithm validation methodology we presented in the previous section.
A. Patient compliance
A key aspect for the validation of the proposed algorithm is patient response rate and compliance with the prospective study. As demonstrated in Table 3, 50% of the patients enrolled show 50% adherence to the DSQ across their entire usage period. Additionally, patients appear to be more compliant in providing answers in the first 6 months of the study while they tend to reply less over time. Moreover, as shown in Fig. 4, median adherence seems to vary among pilot sites with TUK presenting 56% overall median adherence, MST 60% and GEM 35%. Such an effect could be explained by the fact that GEM patients are older and thus face challenges with digital literacy. Moreover, they can be in a more progressed disease phase that contributes to further reducing engagement and compliance. Research nurses have reported such observations during enrolment and follow-up visits. Additional analyses on the education level are outside the scope of this work and might be further explored in the future.
Table 3: DSQ Adherence over time
DSQ Adherence (%) (median, q1, q3)
| Observation Period | GEM (n=86) | MST (n=71) | TUK (n=75) | All (n=232) |
|---|---|---|---|---|
| 6 months | 55.0 (16.0, 87.4) | 80.3 (45.7, 95.0) | 82.2 (69.3, 91.0) | 76.7 (38.0, 91.3) |
| 1 year | 41.4 (8.1, 74.8) | 66.3 (29.9, 93.7) | 77.9 (55.8, 89.6) | 63.8 (26.6, 89.6) |
| All period | 34.8 (6.3, 69.2) | 59.6 (18.4, 86.1) | 56.4 (30.4, 82.4) | 49.8 (17.8, 79.3) |
Regarding the COPD Symptoms questionnaire, 50% of patients in both the entire cohort and the single pilot sites were 100% adherent in providing answers for the specific symptom changes once replying positively to symptom worsening through the DSQ.
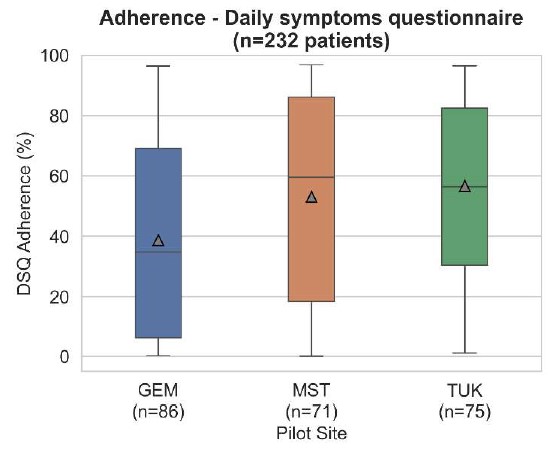
Fig. 4. DSQ adherence per pilot site for the entire observation period
B. Exacerbation detection, frequency and duration
In Table 4, COPD exacerbations are reported as COPD Exacerbation Onset states. Overall, 71 patients (31% of the entire cohort) presented exacerbation events during the observation period. The algorithm detected a total of 238 events, with 149 occurring in 32 patients at MST pilot site, 68 in 32 patients at GEM, and only 21 events in 7 patients at TUK. At the same time, multiple COPD Unstable states are detected for a total number of 116 patients.
Exacerbation events appear to be more frequent for some patients compared to others. As can be seen in Fig. 5, most patients experienced a single exacerbation event while the number of patients with higher exacerbation frequency is limited. The exacerbation frequency profile differs significantly between pilot sites, with MST including the most frequently exacerbating patients while TUK the least ones. At GEM, instead, most patients experienced between 1 and 3 exacerbations. This variation in exacerbation rates across sites may be due to differences in patient populations and care models.
Table 4: COPD Exacerbations per pilot site
Exacerbations (Patients)
| Exacerbation State | GEM | MST | TUK | All |
|---|---|---|---|---|
| COPD Exacerbation Onset | 68 (32) | 149 (32) | 21 (7) | 238 (71) |
| COPD Unstable | 205 (49) | 337 (45) | 70 (22) | 612 (116) |
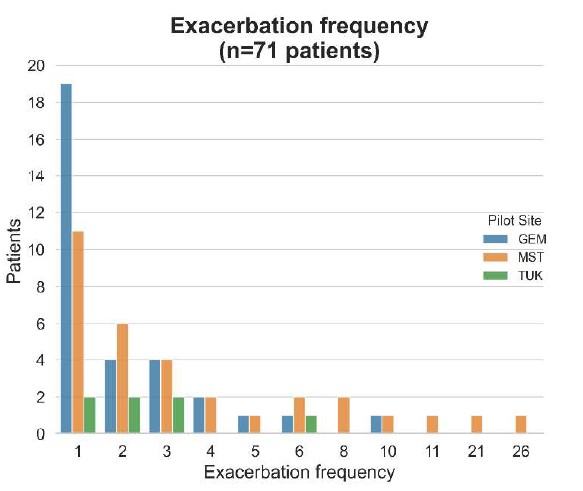
Fig. 5. Exacerbation frequency distribution per pilot site
As demonstrated in Fig. 6, most exacerbation events get concluded within 15 days while others can last from 30 and up to 65 days. More specifically, the mean duration for the entire cohort is 11 days and remains comparable across pilot sites; 10 days at MST, 11 at GEM and 14 at TUK. As detailed in the previous section, the algorithm ensures that when patients do not report on their condition daily, event duration gets limited to a maximum of 30 days. However, it was observed that few exacerbations exceeded this duration, comprising outliers, and were reported before the 30-day limit applied to the algorithm. These observed prolonged exacerbations were the reason why we needed to add such time limit. At all pilot sites there are events that last only 5 days, duration that holds for the minimum exacerbation duration by definition according to the COPE-III protocol considering 4 days in Exacerbation state and 1 day in Unstable state. Interestingly, the more patients are compliant in reporting their health condition, the more accurate seems to be the event detection mechanism. This can be observed in Fig. 6 for MST site.
C. Algorithm validation at GEM pilot site
As discussed previously, the proposed algorithm detects COPD exacerbation events based on patient reported symptom changes on daily basis.
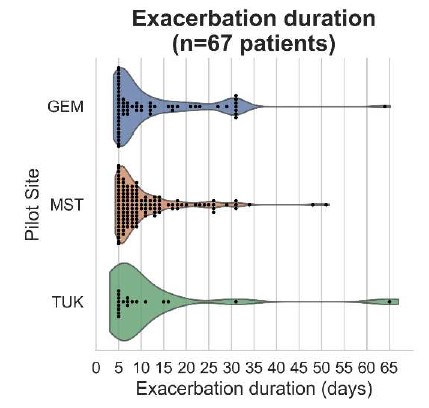
Fig. 6. Exacerbation duration distribution per pilot site.
As shown in Table 5 at GEM pilot site 26 patients present in total 64 clinical events such as emergency accesses, hospitalizations, death, considering the entire observation period. 59% of the reported events correspond to hospitalizations, 34% emergency accesses and only 6% death. Please note that each patient can fall in more than one event categories and therefore the total patient number represents the distinct number across all event types.
Table 5: Events at GEM pilot site
| Event type | Events | Patients (n=86) |
|---|---|---|
| Emergency access | 22 (34.4%) | 15 (17.4%) |
| Hospitalization | 38 (59.4%) | 19 (22.1%) |
| Death | 4 (6.2%) | 4 (4.7%) |
| Total | 64 | 26 (30.2%) |
To validate whether an exacerbation event detected by the algorithm corresponds to an actual health deterioration incident, we match COPD states including Exacerbation Onset and Unstable to reported clinical events under the condition that both events occurred within a 30-day temporal window with the algorithm-detected states preceding the clinical events. Such an assumption allows for the validation of early detection of acute clinical events that usually remain less frequent compared to moderate ones. It is important to note that the COPE-III protocol aims to capture mainly moderate events and prevent exacerbations at an early stage. In this work, we do not validate moderate events since the required medication data for performing such a task is currently not available.
In Table 6 we report all algorithm detected states matched to clinical events. Considering both Unstable and Onset states, the algorithm is able to detect 22 disease deterioration events and more specifically 46% of the emergency accesses and 32% of hospitalizations reported at the GEM pilot site. Considering that the adherence of patients has been as low as 33%, such results are promising. On the contrary, death events are not matched to detected COPD states as adherence to symptoms questionnaire has been on average only 13%. Finally, it is worth mentioning that 4 additional clinical events were matched to a positive answer to symptoms change (DSQ) without providing further answers to COPD symptoms questionnaire and thus they are not included in this analysis, but they could be accounted for in future work.
Table 6: Algorithm detected states matched to events at GEM pilot site
| Event type | Unstable | Exacerbation Onset | Events |
|---|---|---|---|
| Emergency (n=22) | 7 (32.0%) | 3 (14.0%) | 10 (45.5%) |
| Hospitalization (n=38) | 9 (24.0%) | 3 (8.0%) | 12 (31.6%) |
In Fig. 7 we also analyzed the time interval at which patients declared symptom changes and how those are associated to clinical events. It is observed that detected COPD Unstable states can be crucial in detecting disease deterioration more often than COPD Exacerbation Onset states. Such findings, confirm that data availability assumptions can be further explored with the aim to better define a minimum sufficient patient input to detect exacerbation events timely and more efficiently.
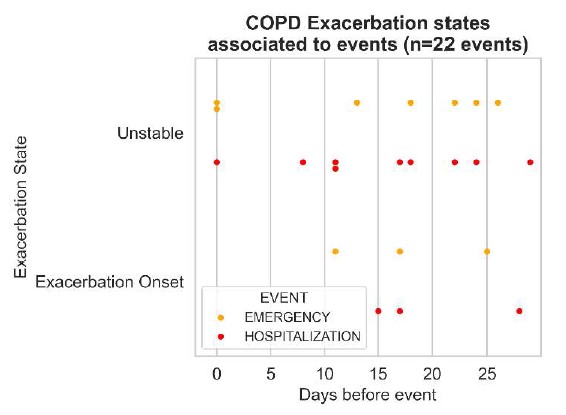
Fig. 7. Exacerbation states associated to events at the GEM pilot site.
D. Exacerbation profiles at GEM pilot site
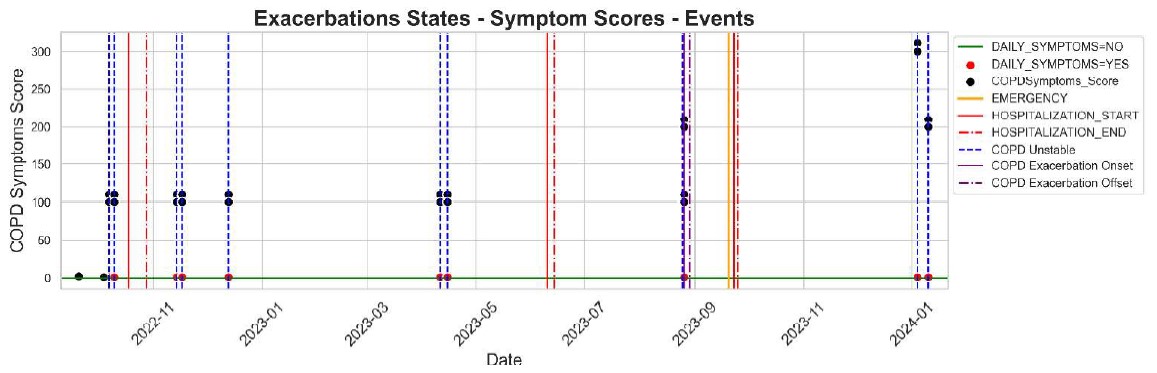
In Fig. 8 we provide an example of a patient that presents clinical events at the GEM pilot site. The patient gets through three hospitalization events and one emergency access before the last hospitalization. The first hospitalization event (in red) is preceded by a COPD Unstable state (in blue) while the emergency access (in yellow) and successive hospitalization event (in red) is preceded by an Exacerbation Onset state (in purple). The second hospitalization event is not matched to change in his COPD symptoms. This example showcases in a direct manner the potential of the proposed system and at the same time its limitations in engaging the patient in using the technology. Further evaluation is needed to explore these limits and to tackle them.
IV. DISCUSSION
A. Challenges and limitations
The protocol and algorithm design present limitations in accurately capturing the diverse manifestation of COPD exacerbations and the unique per patient symptomatology. The pre-set score thresholds and scoring system, while based on expert judgment, may not adequately capture all possible symptom changes or their relative clinical significance. It also seems that the algorithm is too sensitive for some patients as facing over 10 COPD exacerbations per year is clinically not plausible. Additionally, some patients’ symptom patterns might not align well with the selected categories present in the DSQ, making it hard to properly classify their condition or detect their deterioration. Therefore, the initial algorithm design and threshold settings will require iterative refinement as more real patient data becomes available. Despite the possibility that the system will not perform optimally in its early implementations, the settings and algorithm will be validated and adjusted continuously as the study progresses.
The algorithm may struggle when patients skip completing the DSQ for several days as it relies on consistent data input. Without consistent data, it becomes difficult to accurately track changes in their condition. Patients naturally do not maintain perfect day-to-day adherence. However, in the initial implementation of the algorithm this was not very well accounted for. The algorithm presented in this paper is refined based on validation during the observational cohort study, to better handle missing data, ensuring more robust performance in real-world conditions where data gaps are common. Obviously, since the system can only analyse data that patients actually input, missing or irregularly completed questionnaires can make it impossible for the algorithm to accurately monitor how symptoms are changing and identify when a patient’s condition is getting worse. Therefore, in RESAMPLE the outcomes of the algorithm are used as a support to HCPs informing them accordingly whenever patients stop reporting on their health condition to recover missing
information and ensure patient safety.
The overall reliability of the system in maintaining consistent performance and accuracy over time remains a significant concern, as any instability, especially if counting only on the automated system, could impact patient care. Technical limitations that include system downtime or malfunctions cannot be guaranteed to be 100% eliminated. Processing delays or challenges in combining data from different sources can also affect the accurate and quick This is the reason why HCPs should consider automated monitoring systems as supportive tools rather than complete replacements for clinical
judgment. While these systems can provide valuable insights, their clinical expertise and direct understanding of patient conditions should remain the primary basis for medical decisions, with the automated system serving as a complementary aid to enhance their professional assessment and care delivery.
Finally, the algorithm validation methodology we presented, aims to assess outcomes in a pragmatic way making
use of the currently available clinical data at one pilot site. An interesting future direction of work could be a comprehensive clinical evaluation on the entire cohort using all pilot clinical data for both severe but especially moderate events.
V. CONCLUSIONS
In this work, we introduced an algorithm, based on the COPE-III protocol, for the real-time identification of COPD exacerbations. Even though data collection in a real-world setting remains challenging, particularly with patient adherence averaging 49.8% across all sites, the developed model has the potential of detecting a major worsening of the health status of a patient based on self-reported data. The timely identification of COPD exacerbations, validated with respect to hospitalizations and emergency accesses, appears to be promising for the actual use and implementation of the algorithm in clinical practice together with the adoption of the RE-SAMPLE platform. However, data availability remains a bottleneck. Adherence is not always high and sufficient to detect these events. Therefore, it is important to establish a comprehensive validation approach using independent clinical data collected in the scope of this study for the enrolled COPD patients. Future work directions may include algorithm improvements to overcome the limitations and barriers
identified in the previous discussion. Additionally, further validation across MST and TUK pilot sites with additional data is required in order to have clear evidence of the robustness of the implemented protocol. More specifically, a benchmark including exacerbation events should be created and carefully validated by medical experts from all three pilot sites. Such benchmark can permit a systematic analysis of the sensitivity and the specificity of the proposed algorithm given certain adherence levels. Refinements to the protocol scoring system might be suggested from an in-depth investigation on the correlation between the detection of exacerbation events and hospital admissions, which should be discussed and tested before their actual deployment. The expansion of the study to the interventional VCP, along with the inclusion of additional
patients and end-users, might be a key factor for the final refinement of the model and its consequent future use.
ACKNOWLEDGMENT
This work is supported by the RE-SAMPLE project that has received funding from the European Horizon 2020 research and innovation program under grant agreement No 965315. This result reflects only the author’s view and the European Commission is not responsible for any use that may be made of the information it contains.
REFERENCES
[1] Global burden of COPD. Respirology, 2016, 21.1: 14-23.
[2] WHO, Chronic obstructive pulmonary disease (COPD) (accessed February 2025)
[3] HILLAS, Georgios, et al. Managing comorbidities in COPD. International journal of chronic obstructive pulmonary disease, 2015, 95-109.
[4] VINIOL, Christian; VOGELMEIER, Claus F. Exacerbations of COPD. European Respiratory Review, 2018, 27.147.
[5] KIM, Victor; AARON, Shawn D. What is a COPD exacerbation? Current definitions, pitfalls, challenges and opportunities for improvement. European Respiratory Journal, 2018, 52.5.
[6] SEEMUNGAL, Terence AR, et al. Time course and recovery of exacerbations in patients with chronic obstructive pulmonary disease. American journal of respiratory and critical care medicine, 2000, 161.5: 1608-1613.
[7] WEDZICHA, Jadwiga A.; SEEMUNGAL, Terence AR. COPD exacerbations: defining their cause and prevention. The lancet, 2007, 370.9589: 786-796..
[8] American Thoracic Society Patient Education Series. of COPD Am J Respir Crit Care Med Vol. 198, P21-P22, 2018. Available online at https://www.thoracic.org/patients/patientresources/resources/copd exacerbation-ecopd.pdf (accessed February 2025)
[9] AGUSTÍ, Alvar, et al. GOLD 2023 executive summary: responses from the GOLD scientific committee. The European Respiratory Journal, 2023, 61.6: 2300616.
[10] TABAK, Monique; OP DEN AKKER, Harm; HERMENS, Hermie. Motivational cues as real-time feedback for changing daily activity behavior of patients with COPD. Patient education and counseling, 2014, 94.3: 372-378.
[11] BEINEMA Tessa, et at. Tailoring coaching strategies to users’ motivation in a multi-agent health coaching application. Computers in Human Behavior, 2021, 121: 106787.
[12] JUNG, Tony; VIJ, Neeraj. Early diagnosis and real-time monitoring of regional lung function changes to prevent chronic obstructive pulmonary disease progression to severe emphysema. Journal of Clinical Medicine, 2021, 10.24: 5811.
[13] OLIVEIRA, A. S., et al. Identification and assessment of COPD exacerbations. Pulmonology, 2018, 24.1: 42-47.
[14] COOPER, Christopher B., et al. Remote patient monitoring for the detection of COPD exacerbations. International Journal of Chronic Obstructive Pulmonary Disease, 2020, 2005-2013.
[15] TIWARI, Abhishek, et al. Remote copd severity and exacerbation detection using heart rate and activity data measured from a wearable device. In: 2021 43rd Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC). IEEE, 2021. p. 7450-7454.
[16] BELLOU, Vanesa, et al. Prognostic models for outcome prediction in patients with chronic obstructive pulmonary disease: systematic review and critical appraisal. Bmj, 2019, 367.
[17] SHAH, Syed Ahmar, et al. Exacerbations in chronic obstructive pulmonary disease: identification and prediction using a digital health system. Journal of medical Internet research, 2017, 19.3:e7207.
[18] SANCHEZ-MORILLO, Daniel; FERNANDEZ-GRANERO, Miguel Angel; JIMÉNEZ, Antonio León. Detecting COPD exacerbations early using daily telemonitoring of symptoms and k-means clustering: a pilot study. Medical & biological engineering & computing, 2015, 53: 441-451.
[19] FERNANDEZ-GRANERO, Miguel Angel; SANCHEZMORILLO, Daniel; LEON-JIMENEZ, Antonio. An artificial intelligence approach to early predict symptom-based exacerbations of COPD. Biotechnology & Biotechnological Equipment, 2018, 32.3: 778-784.
[20] WANG, Chenshuo, et al. Comparison of machine learning algorithms for the identification of acute exacerbations in chronic obstructive pulmonary disease. Computer methods and programs in biomedicine, 2020, 188: 105267.
[21] LENFERINK, Anke, et al. A self-management approach using self-initiated action plans for symptoms with ongoing nurse support in patients with chronic obstructive pulmonary disease (COPD) and comorbidities: the COPE-III study protocol. Contemporary clinical trials, 2013, 36.1: 81-89.
[22] LENFERINK, Anke, et al. Exacerbation action plans for patients with COPD and comorbidities: a randomised controlled trial. European respiratory journal, 2019, 54.5.
[23] SLOOTS, Joanne, et al. Adherence to an eHealth selfmanagement intervention for patients with both COPD and heart failure: results of a pilot study. International journal of chronic obstructive pulmonary disease, 2021, 2089-2103.
[24] TABAK, Monique, et al. A telehealth program for selfmanagement of COPD exacerbations and promotion of an active lifestyle: a pilot randomized controlled trial. International journal of chronic obstructive pulmonary disease, 2014, 935-944.
[25] ACEBES, Alberto, et al. Re-sample platform for training and use of copd exacerbation risk prediction models. European Respiratory Journal, 2024, 64.
[26] LEHMANN, Jakob, et al. Federated learning in multi-center, personalized healthcare for copd and comorbidities. In Proceedings 18th International Conference on Health Informatics (HEALTHINF 2025), 20-22 February 2025, Porto, Portugal, 2025. SCITEPRESS
[27] PAGLIARI, Giulio, et al. A multiple source data collection and integration paradigm for the creation of a dynamic COPD data mart. In Proceedings 18th International Conference on Health Informatics (HEALTHINF 2025), 20-22 February 2025, Porto, Portugal, 2025. SCITEPRESS
[28] Healthentia SaMD, “Healthentia Software as Medical Device”. Available at https://healthentia.com. Accessed on 2/20/2025
[29] PNEVMATIKAKIS, Aristodemos, et al. Risk assessment for personalized health insurance based on real-world data. Risks, 2021, 9.3: 46.
[30] KYRIAZAKOS, Sofoklis, et al. Benchmarking the clinical outcomes of Healthentia SaMD in chronic disease management: a systematic literature review comparison. Frontiers in Public Health, 2024, 12: 1488687.
[31] NR, Anthonisen. Antibiotic therapy in exacerbations of chronic obstructive pulmonary disease. Ann Intern Med, 1987, 106.2:196-204.
[32] RODRIGUEZ-ROISIN, Roberto. Toward a consensus definition for COPD exacerbations. Chest, 2000, 117.5: 398S-401S. Fig. 8.